Which of the Following Is an Electrolyte Solution
When we dissolve urea in water it does not get ionized to generate its constituents ion because it is an organic compound which does not dissociate to generate ions. NaCl melts at 800C.
What Are Some Examples Of A Strong Electrolyte Quora
Acetic acid is a weak electrolyte.

. The distinguishing characteristic of all electrolyte solutions is that they A contain molecules. Na 2 CO 3. This is just one of the solutions for you to be successful.
Hii i think that electrolyte solution is the one that has ions in water D. Calculate i the pressure when boiling begins ii the composition of each component in the. Precipitate in water O D.
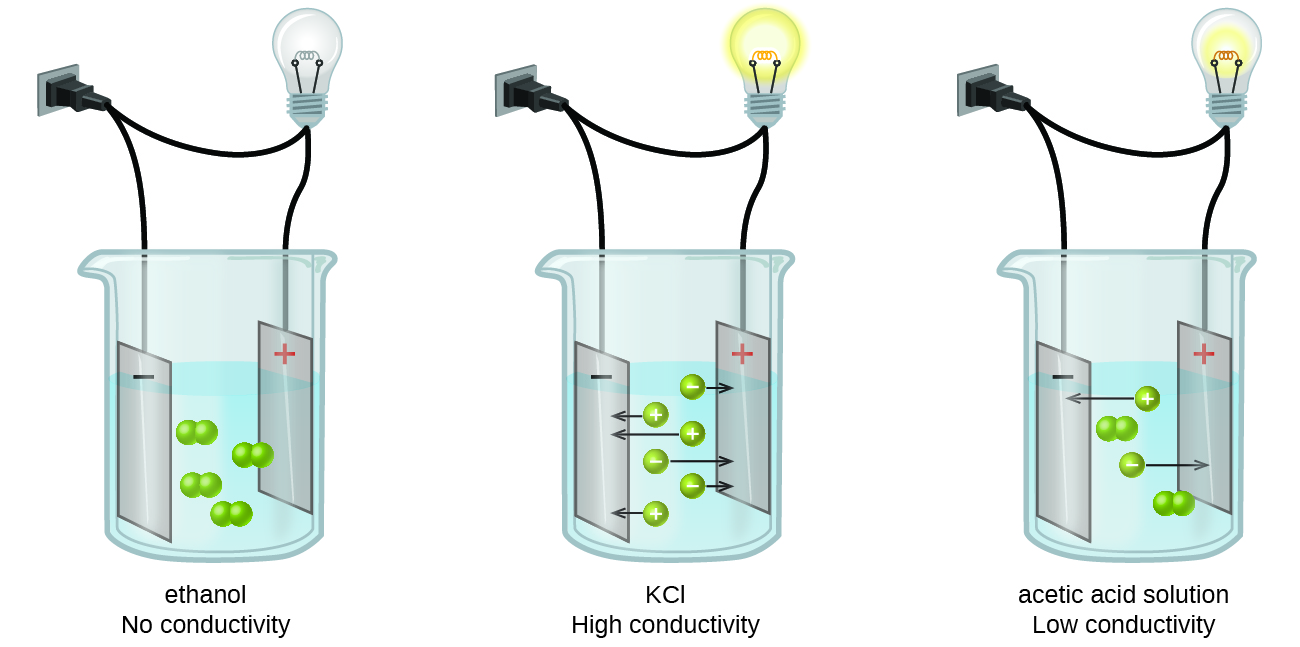
Benzene and toluene form nearly ideal solutions. Glucose ethanol and urea are non-electrolytes. Na 2 SO 4.
Which of the following is a strong electrolyte. 338 10-3 M E. Oil in water B.
Which of the following solution is non-electrolyte. Na2SO4 is strong electrolyte Salts are strong View the full answer Transcribed image text. Therefore urea is an example of non-electrolyte.
Sodium also helps nerve and muscle cells interact. The solution is boiled by reducing the external pressure below the vapour pressure. 625 10-2 M B.
Molecules in water O C. It is difficult to attain and maintain its melting point. Which substance is not an electrolyte.
422 10-7 M D. Starting with a 135 M HCl stock solution five standard solutions are prepared by sequentially diluting 500 mL of each solution to 1000 mL. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state.
H 2 SO 4. Consider an equimolar solution of benzene and toluene. Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions.
Which of the following is an electrolyte solution. Sand in water C. Following Would Form An Electrolyte SolutionWhich Of The Following Would Form An Electrolyte Solution Yeah reviewing a books which of the following would form an electrolyte solution could grow your close associates listings.
C react with other solutions. Oct 24 2007. At 20 c the vapour pressures of pure benzene and toluene are 99 kpa and 29 kpa respectively.
Certain acids are considered to be strong which means they are dissociated 100 in solution. Which of the following is an electrolyte. It has been scientifically proven that 100g of grapefruit contains 135g of potassium electrolyte.
Oxalic acid NaHCO 3. Sodium sulfate Na2SO4 sulfurous acid H2SO3 nitrous acid HNO2 sucrose C12H22011 ammonia NH3. Go back to Phy classroom.
Correct option is A Electrolyte is a substance that dissociates into ions in a solution and acquires the capacity to conduct electricity. D always contain acids. As understood skill does not recommend that you have.
Glucose ethanol and urea are non-electrolytes. Which of the following would form an electrolyte solution. Keeping it similar to the general acid properties Arrhenius acid also neutralizes bases and turns litmus paper into.
Sugar in water D. Acetic acid is a weak electrolyte. State which of the following solutions are weak electrolytes dil.
Option A Sodium metal is manufactured by the electrolysis of fused sodium chloride mixed with KCl and KF. A sodium sulfate B ethyl alcohol C oxygen D sucrose. Based on the solubility rules which one of the following compounds should be insoluble in water.
Sodium concentration affects serum osmolality and extracellular fluid volume. 169 10-4 M C. You ought to memorize this list because almost every other acid is weak.
Chloride helps maintain osmotic pressure. Frozen pure water O B. Which of the following is an electrolyte when dissolved in distilled water.
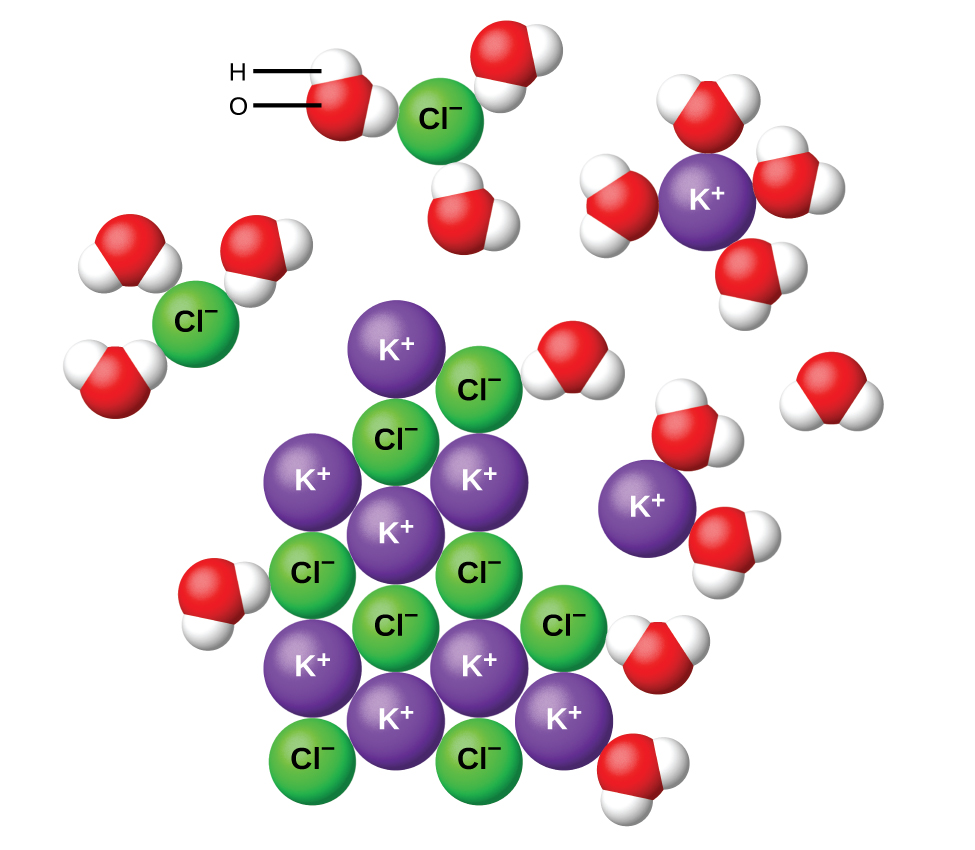
Which of the following is a strong electrolyte in aqueous solution. Electrolytic substances are classified as strong or weak according to how readily they dissociate into conducting ions. Electrolysis is the dissociation of an electrolyte into ions at the electrodes by the passage of electric current.
So KCl and KF are mixed to lower the melting point of NaCl to about 600CKCl and KF are themselves not electrolysed under the voltage. What is the concentration of the final solution. Glucose Ethanol and Urea are examples of non-electrolyte.
Sodium and chloride the major electrolytes in extracellular fluid exert most of their influence outside the cell. The substances whose aqueous solutions allow the passage of electric current and are chemically decomposed are termed electrolytes. Which one of the following is not an electrolyte.
During the electrolysis of silver nitrate solution silver gets deposited at the cathode while the anode loses. Electrolytic substances are classified as strong or weak according to how readily they dissociate into conducting ions. Glucose ethanol and urea are non-electrolytes.
Lons in water SUBM PREVIOUS 2 See answers Advertisement Advertisement kikizzzz kikizzzz Answer.

Lesson Explainer Galvanic Cells Nagwa
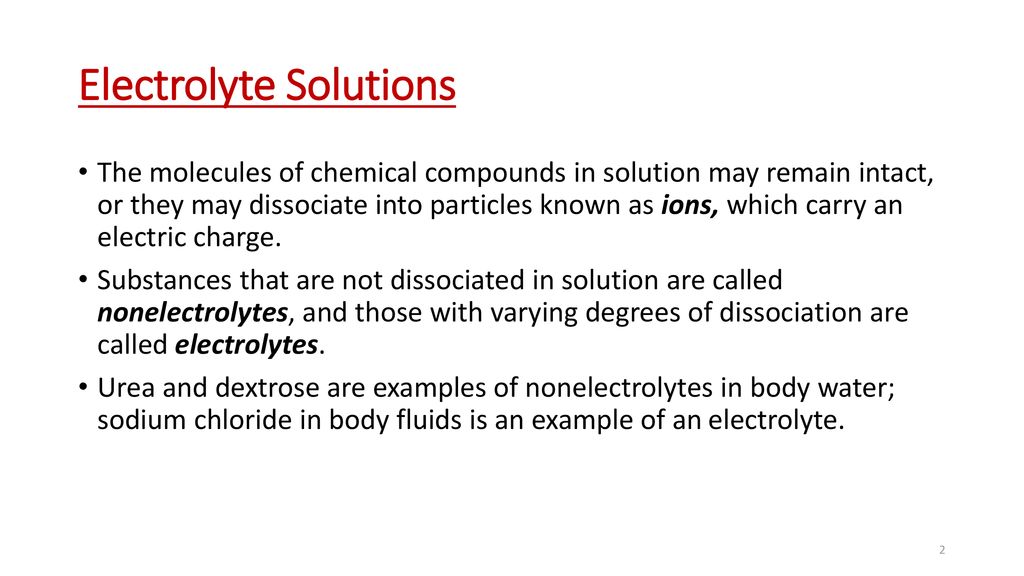
Electrolyte Solutions Milliequivalents Millimoles And Milliosmoles Ppt Download

Electrolyte And Nonelectrolyte Solutions Introduction To Chemistry

9 Electrolyte Examples In Daily Life Studiousguy
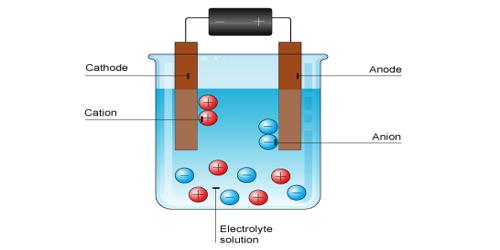
Mechanism Of Electrolytic Conduction Qs Study

Nonelectrolytes Vs Electrolytes Lists Solutions Compounds Video Lesson Transcript Study Com
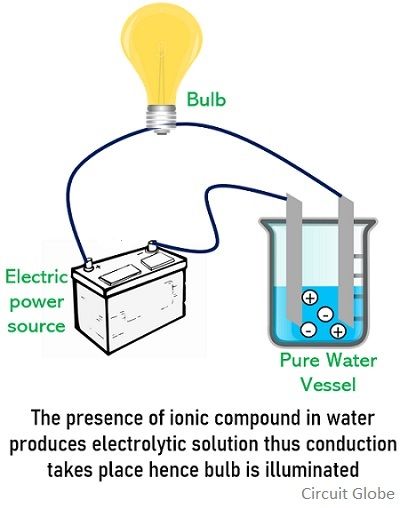
Difference Between Electrolytes And Nonelectrolytes With Comparison Chart Circuit Globe

What Is Battery Electrolyte And How Does It Work Dragonfly Energy
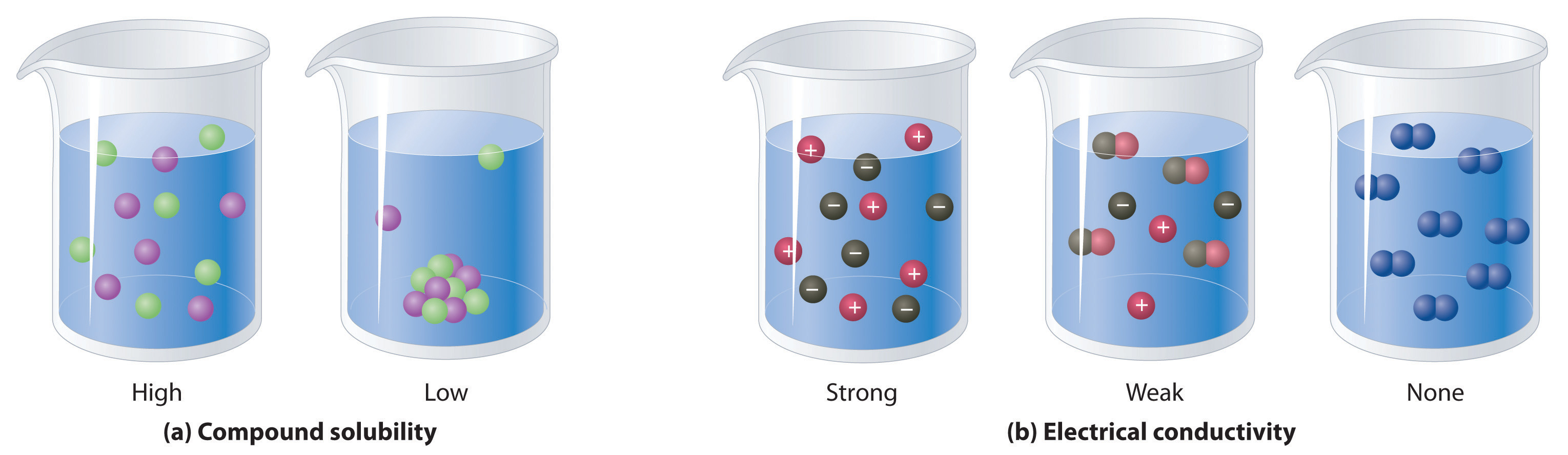
4 1 General Properties Of Aqueous Solutions Chemistry Libretexts

Conductivity Of Electrolytes Demonstration Chemdemos

In An Attempt To Demonstrate Electrical Conductivity Through An Electrolyte The Following Apparatus Was Set Up Which Among The Following Statement S Is Are Correct 1 Bulb Will Not Glow Because The Electrolyte Is

Question Video Identifying A Nonelectrolyte Solution Nagwa

Electrolytes And Nonelectrolytes Chemistry For Non Majors

Electrolysis Electrolytic Cells And Electrolysis Process Examples Videos